

Specialty Care
Infusion treatments, whether at home, in a healthcare clinic or other non-hospital setting, require highly trained Specialty Care nursing and pharmacy support. Trust becomes essential for these higher-risk infusion therapies that require ongoing care.

Clinical Nutrition
Nutrition plays an essential role in sustaining quality of life for those who require care for complex conditions. Pentec Health provides personalized clinical nutrition solutions and superior community-based care.

Disease States
Managing the multifaceted needs for a variety of conditions requiring clinical nutrition and specialty care therapies, services and products. This includes patients with kidney disease, kidney failure, chronic, non-healing wounds, gastrointestinal conditions, rare metabolic disorders, chronic pain, muscle spasticity or cancer within or that has metastasized in the liver.

Continuing Education
By leveraging decades of clinical expertise, we are able to successfully demonstrate our industry leadership through webinars, speaker programs and research studies. We take pride in sharing valuable insights that enhance clinician education and practice.

Patient Resources
We are committed to supporting patients by empowering them with knowledge and resources needed to navigate their healthcare journey with confidence.

Pentec Health
Pentec Health is a national leader in clinical nutrition and specialty care integrating pharmacy services with clinical excellence to enhance the complex care journey.


Poster Presentations
Below is a compilation of Pentec conducted studies featured at past clinical conferences.
Case Studies
Continuous Cycling Peritoneal Dialysis (CCPD)
Gender: Male
Age: 62
Comorbid Conditions: Diabetes, Hyperlipidemia, Secondary Hyperthyroidism, Hypertension
Criteria for IDPN Service: 3 month average albumin less than 3.5g/dL
Formula IDPN: Baxter LL bag 1.5% Amino Acids in 6000mL
Start of Service: 3/27/2018
Break in Service: None
Discontinued Service: Active
Supplements: Liquacel three times a week
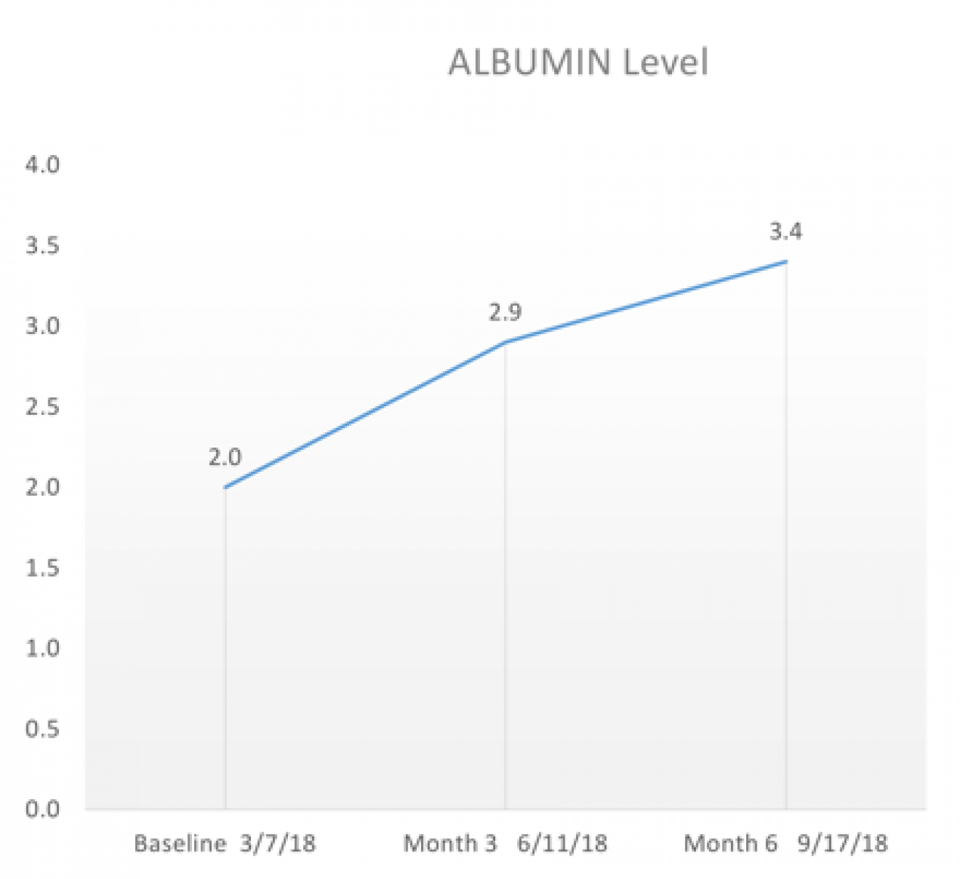
The patient began dialysis on 5/2/17, more specifically this patient started Continuous Cycling Peritoneal Dialysis (CCPD). Patient presented with hypoalbuminemia, with a three month average albumin of 2.4 at time of referral. At that time, the patient was also currently consuming Liquacel 3 times a week to provide 16gms of protein per each serving. The baseline albumin was 2.0 g/ dL. IPN therapy was initiated on 3/27/18 secondary to hypoalbuminenia. Patient is replacing one 6L Baxter dianeal bag with a 6L Baxter IPN bag from Pentec which is providing 90gms of Amino Acids each evening. Due to the increase in the albumin the patient was able to have back surgery in order to repair herniated disks that was causing the patient severe pain which, affected appetite. Patient is still actively receiving IPN and we are awaiting new lab results following the scheduled back surgery.

IDPN Therapy
Gender: Female
Age: 47
Height: 69 in
Target Weight: 55.5 kg
IBW: 65.9 kg
Criteria for IDPN Service: 3 month average albumin less than 3.5g/dL, 84% of her IBW and BMI of 18.12kg/m2
Formula IDPN: 78gms of AA in 490mL
Start of Service: 1/18/2016
Break in Service: No hospitalizations reported
Discontinued Service: 11/2/2016
Supplements: Nepro daily
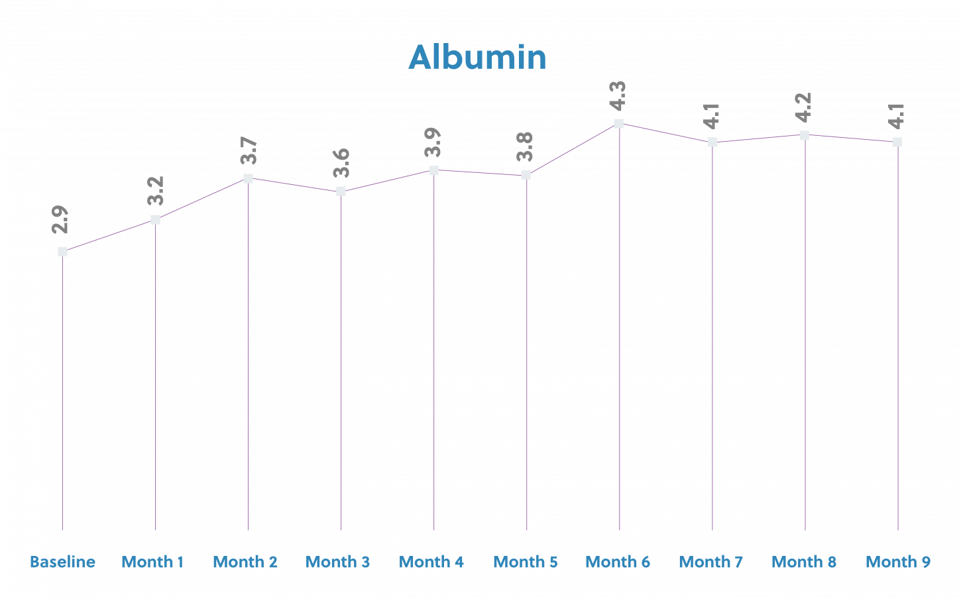
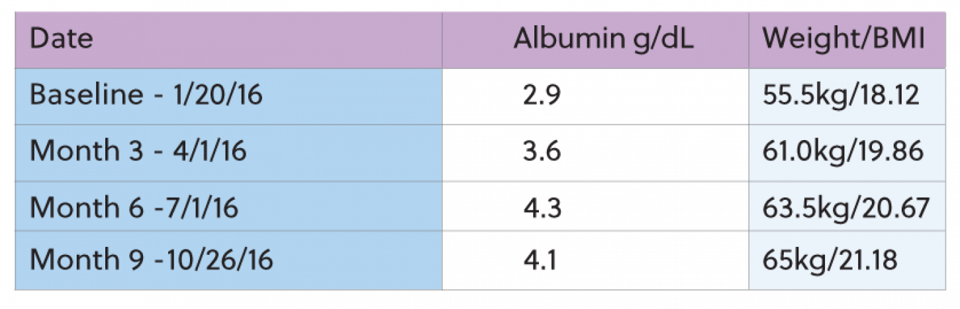
The patient began dialysis treatment 10/11/10. The patient was referred for IDPN therapy because the 3 month average albumin was less than 3.5g/dL and the current albumin was 2.9g/dl. The patient's weight at the time of referral for IDPN was 55.5kg with a BMI of 18.12 and 84% of the ideal body weight. The patient started IDPN with 78gms of amino acids and maintained the same prescription for 9 months while receiving IDPN. During the 9 months that the patient was on IDPN, weight and albumin steadily increased. The IDPN was stopped in October and the patient was able to maintain the albumin level for several months until the point of surgery to remove the transplanted kidney. After the surgery, the albumin declined slightly, however the decision was made to hold off on restarting therapy.


IDPN Therapy
Gender: Male
Age: 57
Height: 69 in
Target Weight: 60 kg
IBW: 72.7 kg
Comorbid Conditions: Coronary artery disease, Hyperlipidemia, Gastric cancer s/p gastrectomy, Hypertension
Criteria for IDPN Service: 3 month average albumin is 3.17, 82.1% of her IBW and weight loss of 6% x 2 months
Formula IDPN: 96 gm of AA in 830mL. Non-protein Kcals - 743 Kcals.
Start of Service: 2/10/2017
Break in Service: N/A
Discontinued Service: 9/20/2017
The patient began dialysis treatment 8/8/14. The patient originally was referred for IDPN therapy due to a 3 month average albumin of 3.1g/dL with a current albumin of 2.9g/dL. Current weight was 60kg with a BMI of 19.5 and the patient was 82.1% of ideal body weight. The patient also had lost 6% of body weight over the last 2 months, further complicating the protein-calorie malnutrition. The patient started IDPN on a lipid formula due to low body weight and recent weight loss to provide extra calories. During the 6 months that the patient was on IDPN, weight and albumin steadily increased. The physician requested to stop IDPN once the weight and albumin had improved and the patient had ultimately met nutritional goals.


Continuous Cycling Peritoneal Dialysis (CCPD)
Gender: Female
Age: 54
Comorbid Conditions: CHF, Liver failure with failed liver transplant, Hypothyroidism, Depression, Anxiety, Insomnia, CAD, SHPT, Anemia, HTN
Criteria for IPN service: 3 month average albumin less than 3.5g/dL
Formula IPN: IPN On Baxter LL bag
1% AA in 6000 ml (60 g protein per day)
Ongoing IPN since 6/30/16
Liquacel 3 times per week
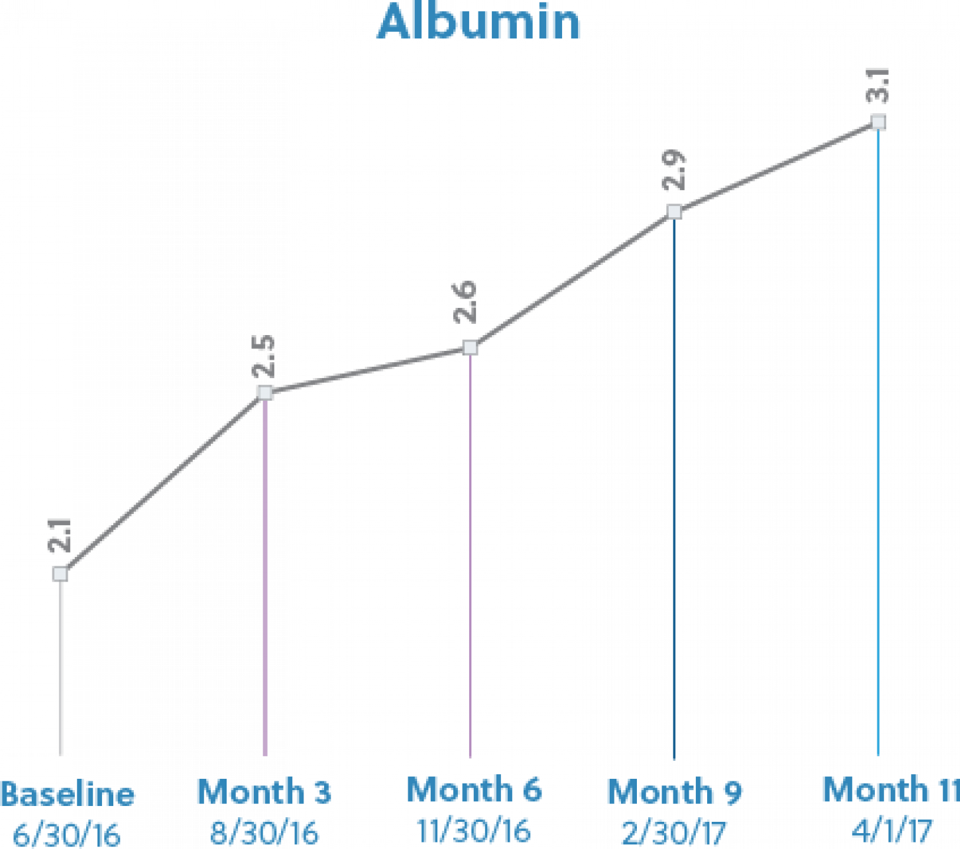
She began continuous cycling peritoneal dialysis (CCPD) 4/25/2011. Her past medical history includes congestive heart failure, liver failure with failed liver transplant, hypothyroidism, depression, anxiety, insomnia, coronary artery disease, secondary hyperparathyroidism, anemia, and hypertension. RD referred patient for IPN in April 2016. Patient presented with chronic hypoalbuminemia with a three month average albumin of 2.53. Her baseline albumin was 2.4 in March 2016 and starting target weight of 56.8 kg (BMI 20.21). She was taking the oral supplement Liquacel (1 oz) 3 times per week at home. IPN therapy was initiated on 6/30/16 secondary to meeting the criteria of hypoalbuminemia. During her time on IPN, she has maintained her EDW, experienced an upward trend in nPNA-PD from 1.9 to 2.32, and maintained adequate Kt/V. Overall, the goal of improving protein status and promoting weight maintenance has been achieved.

Dialysis
Gender: Female
Age: 59
Comorbid Conditions: Diabetes, Congestive Heart Failure, Gastroparesis, Anemia
Criteria for IDPN Service: 3 month average albumin less than 3.5g/dL
Formula IDPN: 60-69kg 90gms of AA in 550mL
Stopped IDPN 9/5/17 Pt received a transplant
Zone protein bar q tx
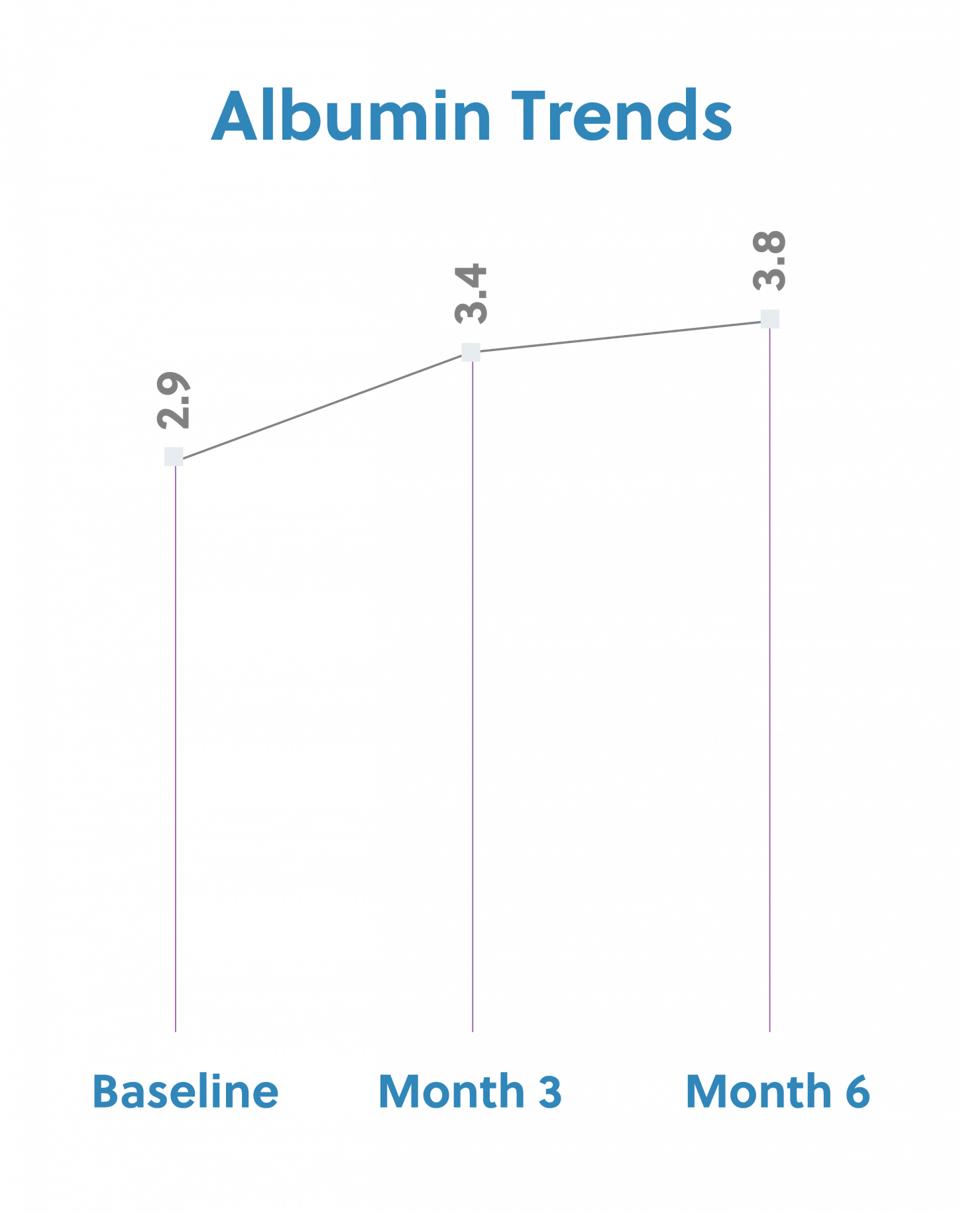
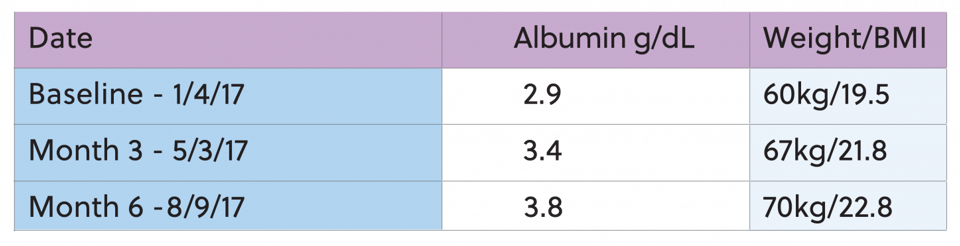
She began dialysis on 9/19/14. Past medical history includes diabetes, congestive heart failure, gastroparesis, anemia. She presented with hypoalbuminemia, with a three month average albumin of 3.4. At this time, she was also currently consuming Zone protein bar 3 times a week at dialysis to provide 14gms of protein. Her baseline albumin was 3.4g/dL and her starting target weight was 78kg. IDPN therapy was initiated on 12/23/16 secondary to meeting the criteria of protein energy wasting. She was listed on the kidney transplant list and was hoping to increase her albumin level in preparation for surgery. Overall, the goal of improving protein status was achieved. The albumin has increased to 3.7g/dL and she received a Kidney transplant.



Dialysis
Gender: Male
Age: 72
Comorbid Conditions: Diabetes, Hypertension, Anemia, Asthma
Criteria for IDPN Service: 6% weight loss x 3 months and 3 month average albumin 3.0g/dL
Formula IDPN: 70kg+96gms of AA in 758mL
Stopped IDPN 10/31/17 for Goals met
Novasource Renal q tx
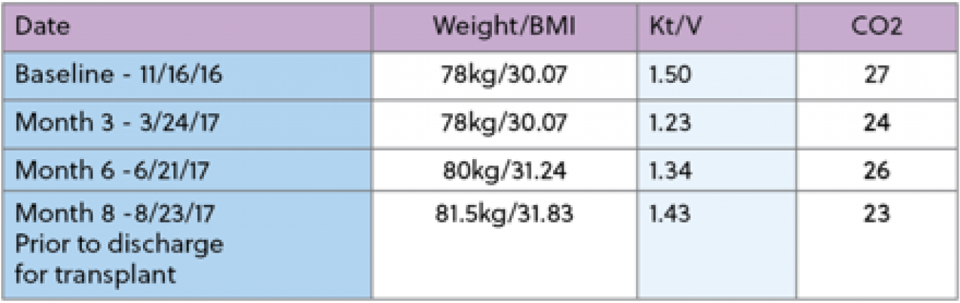
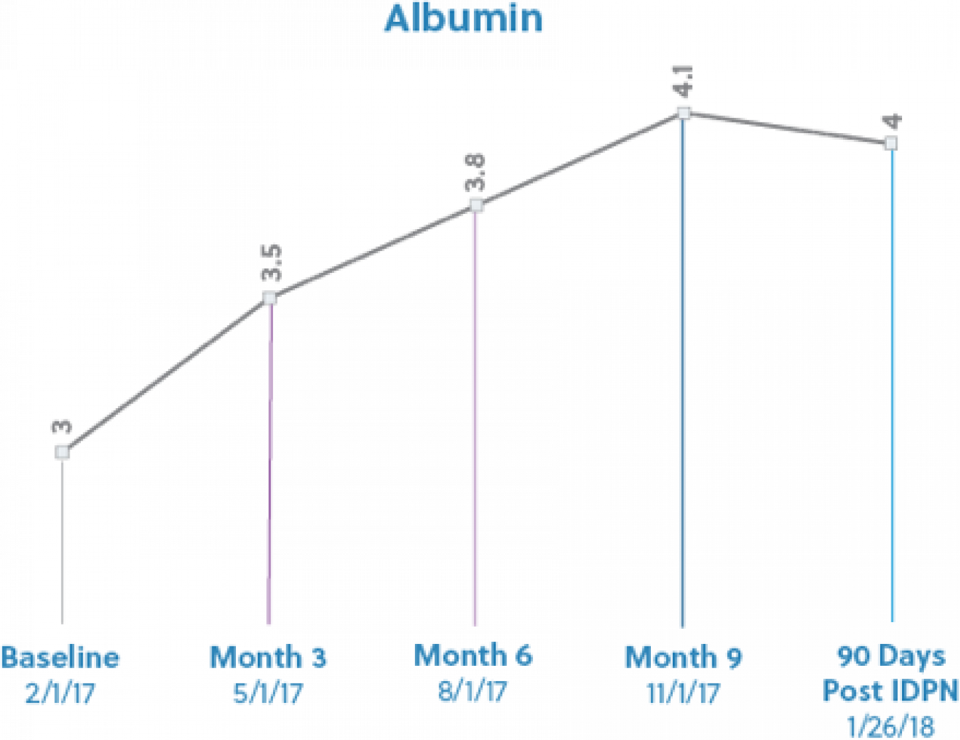
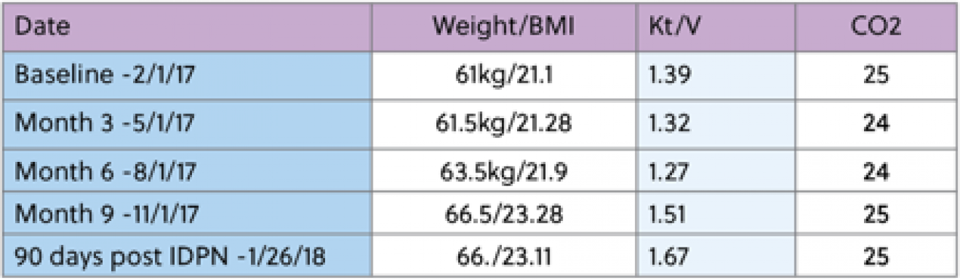
He began dialysis on 10/17/14. Past medical history includes hypertension, type 2 diabetes, asthma, anemia and hypertension. He presented with hypoalbuminemia, with a three month average albumin of 3.0, and a recent weight loss of 6% in the last 3 months. At this time, he was also currently consuming Novasource renal 3 times a week at dialysis to provide 21gms of protein. His baseline albumin was 3.0g/dL and his starting target weight was 60.5kg. IDPN therapy was initiated on 2/10/17 secondary to meeting the criteria of protein energy wasting. During his time on IDPN, he did not experience any further substantial weight loss, Kt/V trended above adequate and hemoglobin trended upward throughout the course of time on therapy. Overall, the goal of improving protein status and prevent further weight loss was achieved. The patient was able to maintain a Albumin level of 4.0g/dL for 3 months and no longer needed the IDPN therapy. Below are a few reports made by the RD at a large dialysis chain.


8/04/2022
Patient reports “that he is really enjoying having extra energy to do things now"
8/04/2022
Per RD "His Albumin has been greater than 4 for the last two months. I have been reluctant to discontinue just because it gives him such a big boost in his mental health as it has to his physical health." RD prefers to monitor and identify if patient is able to maintain status.
8/04/2022
Patient X is doing very well. His albumin is 4.1 he is rocking on. Thanks for helping him to get here and feel wonderful."
8/04/2022
"He is doing great….he is holding on to his 4.0 albumin level….He is rocking on and feeling amazing."

Proudly Quality Accredited
National Quality Approval
The Joint Commission

Accredited Practice Transition
Program With Distinction
American Nurses Credentialing Center
By using this website you accept our privacy policy. Choose the browser data you consent to allow:
